Are genetically modified mosquitos the solution to malaria?
Thinking about known-unknowns and unknown-unknowns.
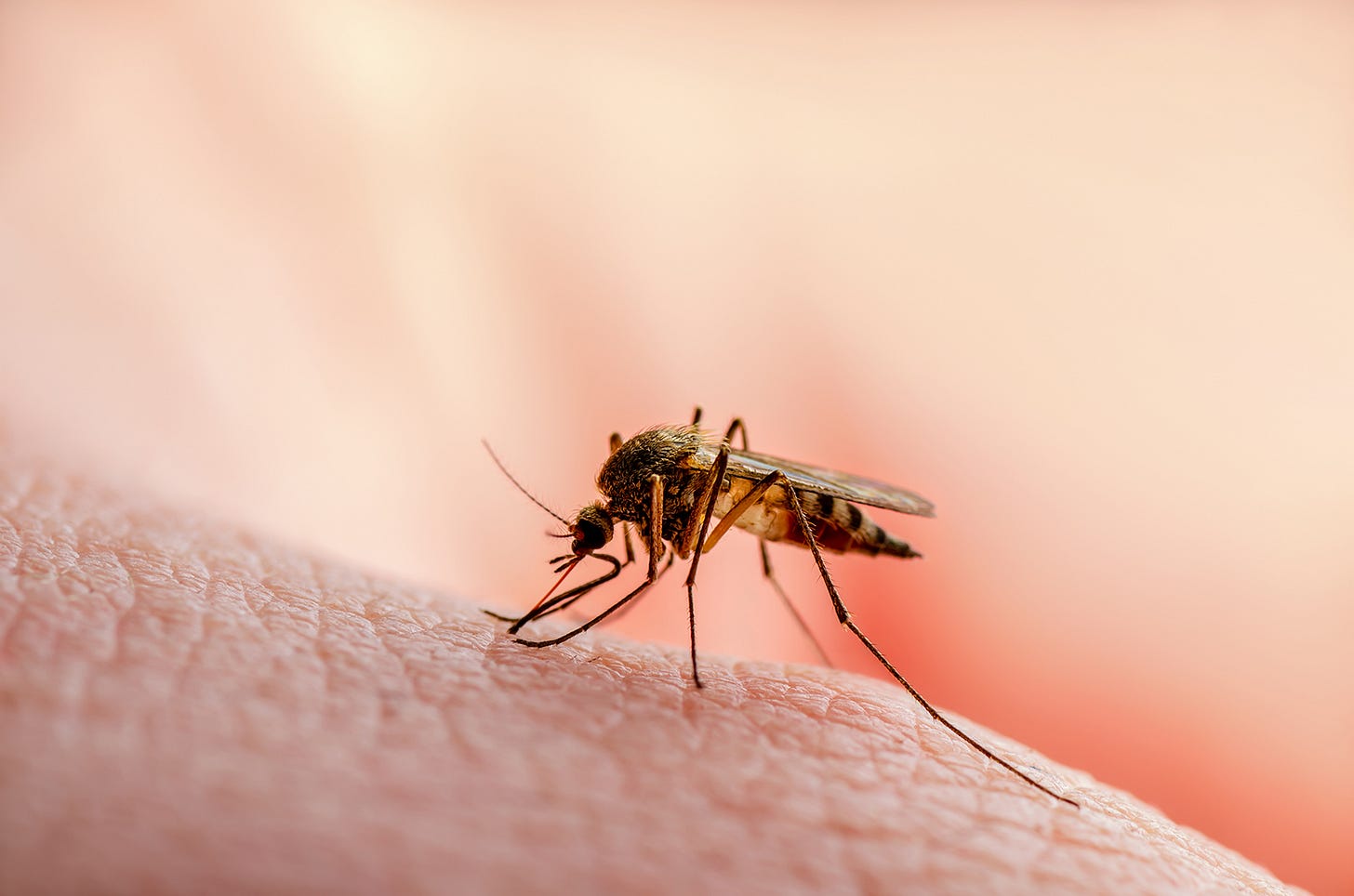
An interesting report in Nature. Oxitec, the biotechnology company, has just completed an open-air trial of its genetically-modified mosquitos. The trial involved releasing five million of them in Florida Keys, a chain of tropical islands off the southeastern tip of the USA.
The five million mosquitos – all males – carried a gene alteration that is lethal to female offspring. The idea is that these males mate with native female mosquitos. The female offspring die. The male offspring carry the gene, so their female offspring die; and so on. The population quickly dwindles. The Florida Keys release was an early stage trial.
Why this murderous plot?
Because, of course, mosquitos are very effective carriers of disease – malaria, dengue, Zika virus, Chikungunya virus, Japanese encephalitis, to name just a few.
This Florida Keys experiment is not the first trial of genetically engineered mosquitos. Others have tried different approaches – making gene edits that do not kill the mosquitos, but reduce their ability to spread malaria, for example.
Mosquitos have been the subject of other cunning plots too. More than a decade ago, participants at TED were famously treated to a demonstration of a smart mosquito-zapping laser. It has subsequently been developed further to create a “phototonic fence” that shoots down mosquitos as they fly above it. To my knowledge (and please contact me if I am missing it), this is still in the development phase.
The problem with clever ideas comes when they turn out not to be! The law of unintended consequences. Australia seems to have a particular track record here. The cane toad (a native of Latin America) was introduced to Australia (and elsewhere) in an attempt to control canefield pests. This neat idea did not work out as planned, and cane toads became a major pest in their own right. Likewise, rabbits were brought from Europe to Australia as a food source, but their population exploded and they overran the country. And as recently as 2012, a well-intentioned government initiative relocated 26 Tasmanian devils to isolated Maria Island… and inadvertently killed off the local penguin population.
These unforeseen ecological consequences show what can happen when we meddle with biology at the visible level… we have a lot less experience of what can happen when that meddling is at the microscopic level, of individual genes. Former U.S. Secretary of Defense Donald Rumsfeld famously said, “… there are known unknowns; that is to say we know there are some things we do not know. But there are also unknown unknowns—the ones we don't know we don't know. And if one looks throughout the history of our country and other free countries, it is the latter category that tends to be the difficult ones.”
There is certainly a lot that we don’t know about gene drives – and we don’t know what we don’t know.
So it’s far too early to conclude that a novel gene drive could play a substantial part in slowing or stopping malaria – or any other mosquito-borne disease. But with half a million children still dying from malaria every year, it is surely worth (carefully) exploring every possible creative solution.
Until then, it is great to see somewhat more familiar solutions becoming available for malaria – the first vaccine, which WHO endorsed for broad use late last year; and others in the pipeline. More on those another time.
GLOBE is an outlet that allows colleagues and friends of the ApiJect Global Initiative to share their thoughts on matters related to global health and their ongoing work. Send questions or responses to media@apijectglobal.com.
To learn more about the ApiJect Global Initiative, visit our website
Twitter | LinkedIn | YouTube